Isotopically Controlled Semiconductors. David Turnbull Lecture. Transcript E. E. Haller. Stolen from Russia germanium research.
Original full pdf file can be downladed from www.osti.gov/energycitations/servlets/purl/923403-ATx11N/
David Turnbull Lecture Transcript E. E. Haller
2005 Materials Research Society Fall Meeting page 1 of 16
Isotopically Controlled Semiconductors
Eugene E. Haller
Department of Materials Science and Engineering, University of California at Berkeley and Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720
---Переходы металл изолятор в зависимости от допирования с помощью нейтронного облучения
---Metal-Insulator Transition with NTD Ge
In discussing the metal-insulator transition with NTD Ge, let us go back to the table of isotopes to consider what happens when Germanium isotopes are exposed to thermal neutrons. Earlier I described the results of this process with Si. Exposing Ge to thermal neutrons is more complicated because there are 5 stable isotopes: 70Ge, 72Ge, 73Ge, 74Ge and 76Ge.
Many research reactors have a special space in which you can place semiconductors to expose them to thermal neutrons. If you expose 72Ge to neutrons, it captures them with a cross-section of about 1 barn (with a barn being equal to 10-24 cm2) and forms the stable isotope 73Ge. Nothing dramatic has happened. Upon neutron capture 73Ge turns into 74Ge, again a stable isotope turning into another stable one. When 74Ge captures a neutron, however, it becomes 75Ge which is not stable. 75Ge decays with an 82 min. halflife< into 75As emitting a ..... If pure, highly enriched 74Ge were placed in a reactor, it would become n-type, because of the As production. Now let's look at 70Ge. This isotope becomes 71Ge upon capture of a neutron which in turn decays with electron capture into 71Ga. The result is Ga-doped Ge. If pure, enriched 70Ge is placed in a reactor, it turns p-type. Natural Ge, of course, produces both p-type and n-type dopants, in a ratio of about three to one, with more acceptors than donors.
My former student, Kohei M. Itoh, now a professor at Keio University in Yokohama, Japan, conducted a very clever experiment based on the above observations. He wanted to study the metal-insulator transition (MIT), which is the transition from semiconductor like to metallic conduction that occurs when semiconductors are doped with increasingly higher amounts of acceptors or donors. At sufficiently high concentrations the dopants form their own band. Once this happens, there is no longer any thermal excitation required to bring the carriers into the conduction band or the valence band for conduction and one observes metallic conduction.
To set up the experiment, Itoh took a slice of our original ultrapure 70Ge and cut it up into many small pieces. He doped 14 of them from low acceptor concentrations to concentrations approaching the metal-insulator transition but not exceeding it. He then took 10 of the samples and doped all of them to different concentrations beyond the metal-insulator transition. He had 14 samples on the insulator side and 10 on the metal conduction side of the metal-insulator concentration. Itoh was able to structure the experiment in this way because the perfectly random impurity distribution reflects uniform neutron flux and uniform isotopic distribution. There is no more perfectly random way of doping than with neutron transmutation! This is the material of choice to study the metal-insulator transition in its really pure form, not affected by doping fluctuations or related problems.
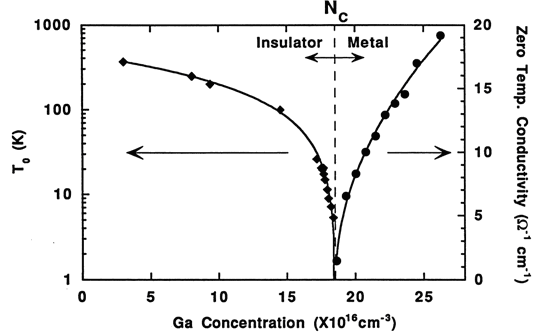
Fig. 6. Extrapolated zero temperature electrical conductivity (right axis) and characteristic temperature T0 (left axis) for variable range hopping for 14 samples with carefully controlled concentrations of 71Ga-doped 70Ge made with neutron transmutation doping. The metal insulator transition (MIT) is found to occur at an acceptor concentration of 1.86x1017 cm-3. [Courtesy K. M. Itoh et al., Phys. Rev. Lett. 77, 4058 (1996)].